Meet GoodGCP
About GoodGCP
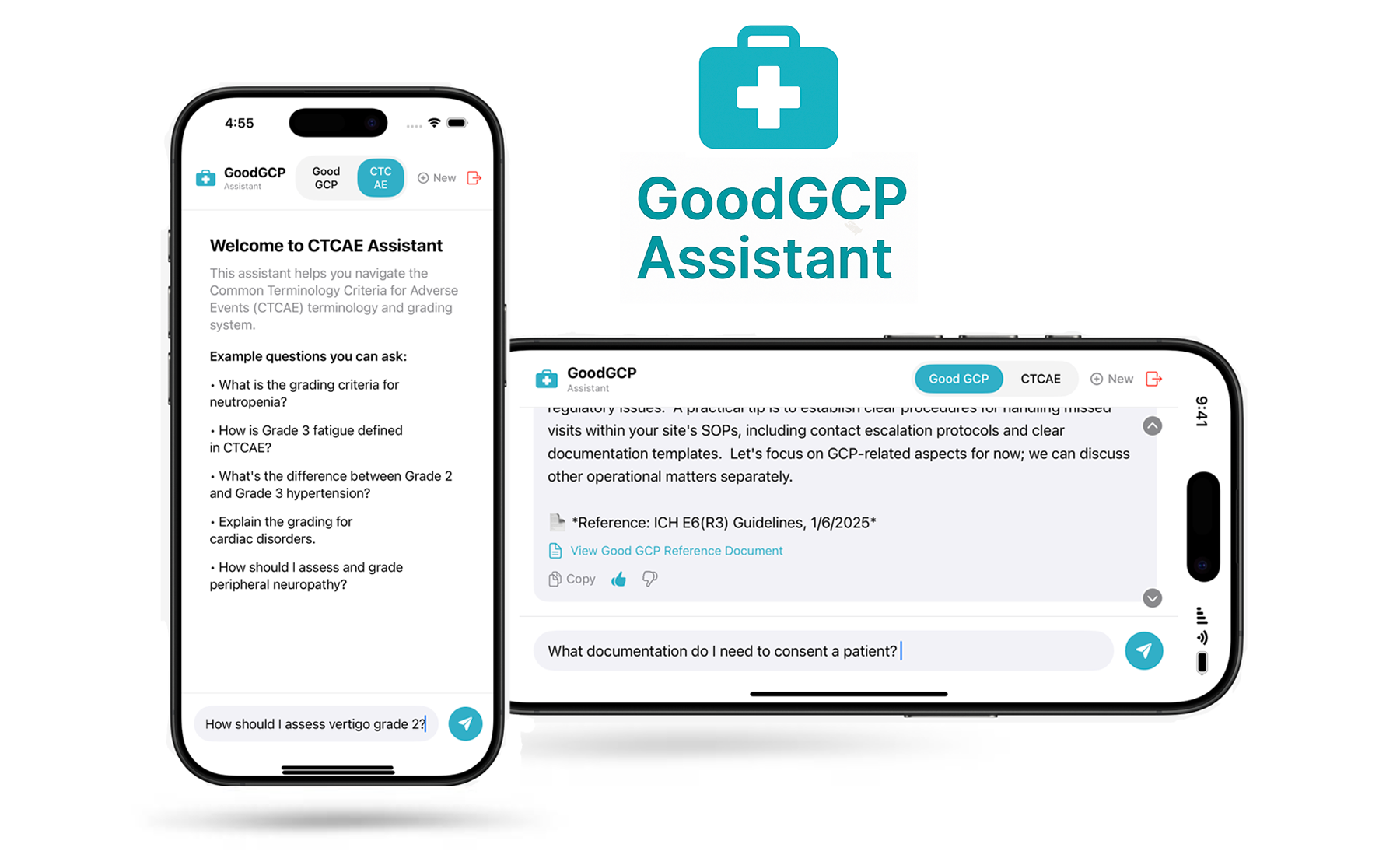
GoodGCP was founded with a simple belief: clinical research teams deserve tools as advanced as the science they support. Today, compliance staff and coordinators spend countless hours searching through hundreds of pages of ICH guidelines, FDA regulations, and CTCAE criteria. We built GoodGCP to change that—an AI assistant that delivers instant, cited answers to complex regulatory questions, freeing teams to focus on patients and science instead of paperwork.
Our mission goes beyond a single app. We’re working to partner with academic medical centers, hospitals, and research networks to build custom enterprise solutions that integrate GoodGCP’s intelligence directly into institutional workflows. From embedding site-specific SOPs to tailoring outputs for therapeutic areas, we aim to become the trusted compliance layer that adapts to each institution’s unique environment.
By combining deep industry insight with cutting-edge AI, we’re not just modernizing compliance—we’re reimagining how research operations can work at scale.
Contact us
Interested in joining the beta? We’d love to hear from you!